Oxbow At Home No Problems Only Solutions

Overview
When two or more kinds of matter are combined, the result is a mixture. Simple mixtures include sand and water, oil and vinegar, nuts and bolts, coleslaw, rocky road ice cream, and trail mix. Sometimes when two (or more) materials are mixed, a different kind of mixture results. For example, when salt and water are mixed, the solid salt seems to disappear in the water. This process is called dissolving; we say that the salt dissolved in the water. This mixture is a solution.
This activity will help students demonstrate what the difference is between a mixture and solution.
Students will also demonstrate different types of solutions through hands-on experiments.
Standards
5th Grade:
S5P1a. Plan and carry out investigations of physical changes by manipulating, separating and mixing dry and liquid materials.
Welcome!
What do you call a tooth in a glass of water?
A one molar solution.
Let's Review!

Mixture: combining two or more substances that can be individually distinguished and easily separated
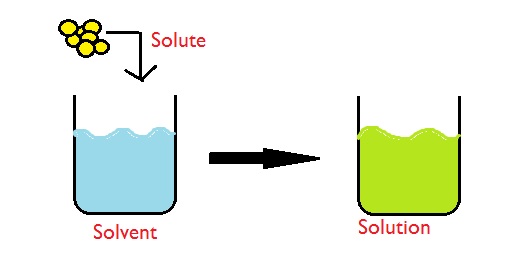
Solvent: a liquid in which a solute is dissolved
Solute: something that can be dissolved into a solvent to form a solution
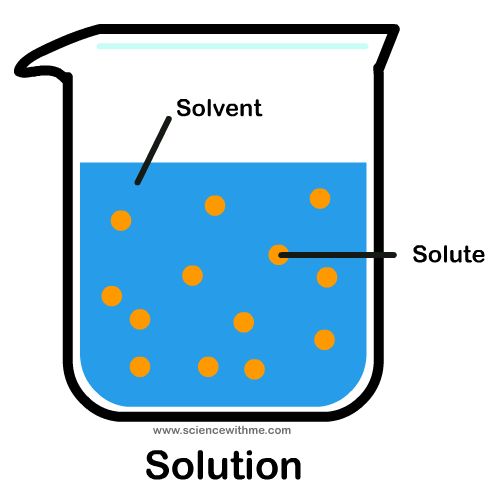
Solution: formed when a solute is dissolved into a solvent
Different Types of Solutions
- Which can be easily separated: a solution or a mixture?
- Basically, a solvent is a liquid that things dissolve in. Can you think of any solvents you may use in your daily life? (Hint: a liquid that you may dissolve something in).
- What is another definition of a solution unrelated to chemistry? Can you think of any solutions to an everyday issue?
Will it Dissolve Experiment
Kool-Aid dissolves, or is soluble in water.
Let’s experiment with some household items to find out: “Will it Dissolve?”
Materials
Material List:
- Plastic cups or glasses (one will do, but the more the better!)
spoon, plastic or metal work best - Pitcher of tap water (or access to a sink to refill glass)
- Table salt, Epsom salt, table sugar, brown sugar, baking soda, corn starch, flour, sand, or dirt (anything you have at home, just adjust data table accordingly)
Data Sheet
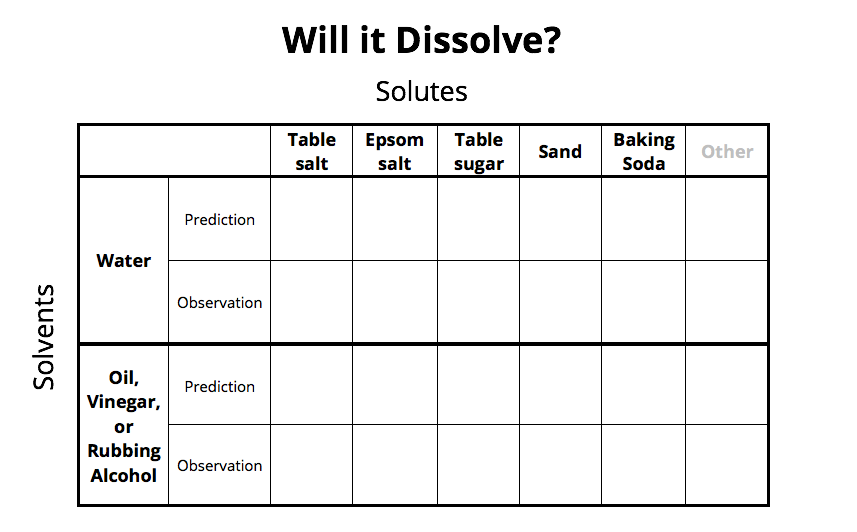
Instructions
- Pour about ½ cup or 4 fluid ounces into a clear cup or glass. If you have multiple cups or glasses, pour one cup for each solute (about 5 or 6 cups depending on how many you will be testing).
- First, make a prediction or an educated guess for what you think will happen when each item or potential solute is added to the cup of water. Write “yes” if you think it will dissolve or disappear, and write “no” if you think it will not dissolve.
- Solutes that dissolve or disappear in the water are “soluble,” while items that settle to the bottom of the cup or float on the top of the water do not dissolve or are “insoluble” in the solvent.
- Use the spoon to scoop one of the solutes into the first cup of water and stir well with the spoon (or a coffee stirrer or a popsicle stick) for a few seconds.
- Record on the data table your observations. You may write what you observed, or write “soluble” if it disappeared into the solvent or “insoluble” if it separated or settled.
- Repeat until you have tested all of the possible solutes that you chose.
You may even want to test an additional solvent such as vegetable oil, white vinegar, or rubbing alcohol (with help from an adult).
Observations
What observations did you make while trying this experiment?
Could you test a different solvent (safely)?
See what happens when you mix two solvents together like water and oil.
What did the thermometer say to the graduated cylinder?
You may have graduated, but I’ve got many degrees.
Why can't we be friends?
Oil vs. Water
Oil vs Water Activity

Materials:
- Clear baby oil
- Clear plastic or glass cup
- Several small cups
- Water
- Food coloring
- Plastic pipettes
The science behind this activity is that water is made of polar chemical bonds and oil is made of non-polar chemical bonds. Basically, this means that water and oil are different and do not attract each other. Even when shaken or stirred, the water and oil will always separate.
What did you notice happening? Draw a picture of what you saw, or observed.
Instructions:
- Fill several small cups with water and add different colors of food coloring to each cup. (Fun tip: you can mix blue and yellow to make green, mix red and blue to make purple, and yellow and red to make orange – don’t forget one or two drops go a long way!)
- Fill one or two cups with about 1 cup of oil (vegetable oil can be used as a substitute for baby oil).
- Use a pipette if you have one, or even a spoon, or just slowly pour a few drops or a spoonful of the colorful water into the cup of oil. You may also choose to use a funnel to avoid making a mess!
- What do you see happening?
- Why do you think the oil “behaves” the way that it does?
The science behind this activity is that water is made of polar chemical bonds and oil is made of non-polar chemical bonds. Basically, this means that water and oil are different and do not attract each other. Even when shaken or stirred, the water and oil will always separate.
What did you notice happening? Draw a picture of what you saw, or observed. Then watch this animated video to take your understanding to the next level with a dance party!
Why can you never trust atoms?
They make up everything!
Let's Get Messy and Make Some Art
Oil and Watercolor Dip Painting
Lucky for us, soap attracts both oil and water. A solution like Dawn dish soap can help attract oil to help clean things like a dirty plate or pan, or even help wildlife affected by harmful oil spills.






